Once all of the above have been put in place, the standardization can take place. A typical measurement routine is as follows:
- Select the correct measurement wavelength.
- Place the blank solution in the holder and set the absorbance display to zero,
- Insert the lowest concentration standard in the instrument and note the absorbance readout.
- Repeat 3 for all standards.
NB: For the standard curve to be appropriate at least three and preferably four standards should be measured.
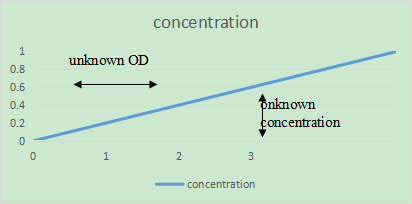
- Plot absorbance vs. concentration ensuring that the line passes through zero.
- Measure the unknown and establish its concentration from reading off the calibration curve.
In practice, the above procedure needs fine-tuning to suit certain conditions. These variations are dealt with below and will be useful to note to produce ideal standard curves.
| Symptom | Cure |
| Standards reading too high | Dilute all standards and samples or decrease the cell path length |
| Samples reading too high | Di Iute samples or introduce more standards |
| Standards reading too low | Increase the path length low of the cell |
| Samples reading too low | Introduce lower low concentration standards or increase cell path length of standards and samples to improve sensitivity |
Linear Calibration Curves
If the calibration curve is linear, subsequent calibration need only be at a single point, e.g. only one standard need be prepared. This means that the Beer-Lambert Law is being obeyed. It is therefore of interest to the user to perform all analysis on a linear calibration curve. This will eliminate the need to prepare large numbers or standards and elaborate calibration plots. So, the dilution of solutions or the reduction of cell path length can often provide the much sought-after linearity.
Summary
For linearity – Dilute the solution or reduce the path length
For sensitivity – Increase path length
Measuring without Standards
There are really two forms of measurement without standards, one acceptable and the other unacceptable.
If a standard curve is constructed and is linear, there will be a factor by which absorbance can be multiplied to obtain concentration.
So, to obtain a factor, take the concentration of any standard, measure its corrected absorbance (e.g. measured after zeroing with a blank), and perform the following calculation.
Concentration / Absorbance=factor
This factor can then be used to perform concentration analysis thus:
- Measure the blank and set it to zero
- Measure the absorbance of the sample
- Multiply the absorbance by the factor to give concentration
This method is legitimate and correct only if the standard curve obeys the Beer-Lambert Law, e.g., the original standard curve from which the factor is taken must be linear.
How not to do it!
Many people use the colorimeter as a color comparator e.g. as a means to check the consistency and continuity of solution color e.g. to ensure that the batch of malt whisky has the same color as the previous batch etc.
In other words, the colorimeter is a sophisticated eye.
lf a solution, after performing a blank, has an absorbance of e.g.0.34 ABU it can be compared with another on the same instrument. If that sample, however, was put on another instrument of the same type, should the absorbance be 0.34 ABU?
The answer is NO!
It could be close but instruments differ and thereby introduce several factors which lead to absorbance values being different:
Filters – not all are the same, they may vary due to the age of the gelatin. The gelatin may be dried and cracked. This could lead to the production of a different max and a different degree of light absorbed or scattered by the filter.
The bulb may be of a different age thereby producing different amounts of energy. This affects the signal-to-noise ratio and the incident intensity.
Detectors may be of different ages and produce slightly different sensitivities.
In summary, if any optical component is slightly different from that of another within a different unit, the optical characteristics of both units will be different. Age is certainly a major contributory factor. So without standardization, a caliber 4mm pocket colorimeter can be used as a color comparator but should not be compared with other units of the same or different type due to the conditions above.
Over time, however, its own optical characteristics will slowly change-making long-term comparisons invalid.
