Analytical instruments often seem as inconspicuous “black boxes”, with vital components well hidden from plain sight. Despite the understandable engineering requirements to shield components from the environment, usually little is given in the way to provide students with a sense of the instrument’s inner workings.
One of the most common applications of colorimeters and the like is for the determination of sulfate content in water. Excessive sulfates have a negative impact on the organoleptic quality of drinking water, and an abrupt change of sulfate concentrations in drinking water can cause a laxative effect on individuals. Not only does the objectionable taste due to excessive sulfates present an issue, but also such water has a negative impact on livestock health and development. Furthermore, states have a very important role in brewing technology, like sulfates, which affect the dryness and astringency of beer and increase bitterness. Another important aspect in brewing technology is the chloride/sulfate balance, which affects the brew’s flavor. The performance of this instrument was evaluated for the determination of sulfate concentration in water, as sulfate analysis is an important water quality parameter and is easily performed in the student lab.
Operating Principle of the colorimeters in the student lab
The underlying principles of pocket colorimeter, and handheld color meter revolve around on absorption and/or scattering of light. A beam of light, with a relatively narrow wavelength distribution, is transmitted through a sample placed in an optically transparent container. If the intensity of the transmitted light is measured, such an instrument is a portable colorimeter and/or a colorimeter. Usually, portable colorimeter operate near-infrared, whereas colorimeters have various spectral ranges (colors) of the source available. Hence, a portable colorimeter measures “cloudiness”, i.e., turbidity of colloidal samples, while the colorimeter can be used to measure the concentration of chromophoric substances. Building on the principles of the portable colorimeter, a color colorimeter measures the intensity of the scattered light, usually at a 90° angle to the incident beam.

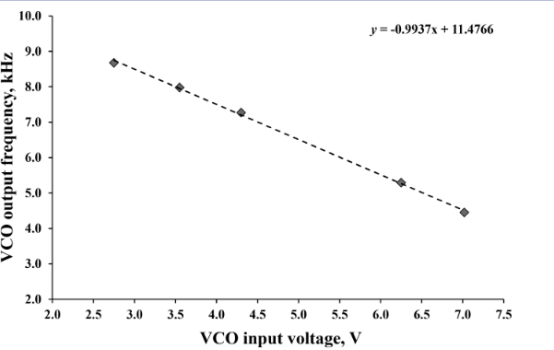
Depending on the intensity of the transmitted, or scattered light, the voltage measured on the midpoint of the selected voltage divider will change accordingly. The voltage divider output is buffered by a non-inverting voltage follower amplifier and fed into a voltage-controlled oscillator based on a 555 timer integrated circuit because purpose-built chips, such as TSL230R, are not widely available. The output of the VCO, effectively a pulse-width modulated (PWM) signal, is attenuated by a voltage divider and fed into a microphone (MIC) input of a PC. Analytical information is extracted from the recorded signal by fast Fourier transform (FFT) analysis, in a stand-alone program written in MATLAB. Absorbance A is calculated according to Beer−Lambert’s law by approximating the light intensity with the frequency of the VCO output according to the following formula: A=log(I0/I)=log(f0/f)
Conclusions
The performance of these caliber 4mm pocket colorimeters instruments was shown to be quite remarkable, considering their simplicity and low cost. The simple construction provides students valuable insight into the operating principles of such instruments and serves as a viable tool for hands-on learning. Not only that, but the colorimeter can be used as a field-capable instrument for water analyses. As such, the instrument has proven itself to be valuable in the determination of sulfate content in drinking water.
